The AICE team at UAB has led Deliverable 7.6, outlining policy recommendations to enhance clinical guidelines for colorectal cancer screening and diagnosis.
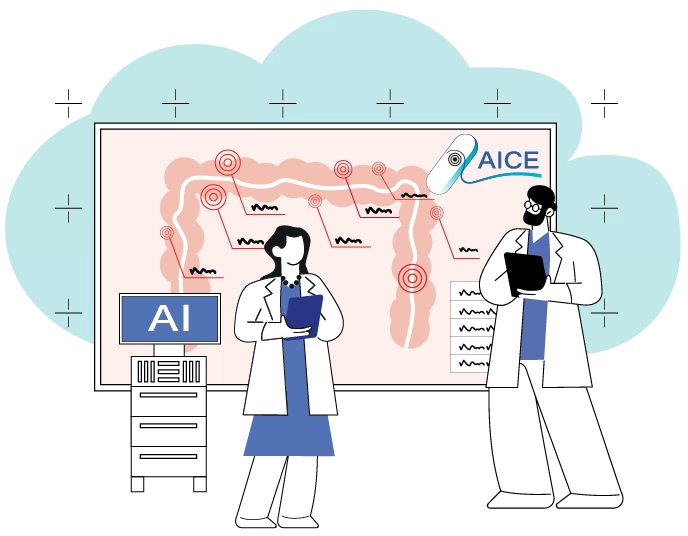
The report brings together scientific evidence with insights from the AICE project. It highlights where current guidance falls short and where new technologies can help. Screening programmes based on faecal immunochemical testing (FIT) followed by colonoscopy are effective but face pressure from demographic factors and limited endoscopy capacity. The recommendations call for risk-stratified pathways, more selective polypectomy, and targeted use of colon capsule endoscopy (CCE), including AI-supported workflows that allow same-day progression to colonoscopy when needed.
For individuals with symptoms, colonoscopy remains the standard investigation, although it is an invasive and resource-intensive procedure. Evidence supports that CCE is a safe and patient-friendly option for low-risk cases. Guidance should address who is suitable, how to manage reinvestigation, and how CCE can be integrated into primary care pathways.
Stronger recommendations are also needed for clear information and shared decision-making, including culturally adapted decision aids and the routine use of patient-reported outcomes. As AI expands across colonoscopy detection and diagnosis, capsule interpretation, imaging, and digital pathology, future guidelines should establish standards for validation, ethics, and workforce training to ensure the safe and equitable use of these technologies.
Coming Soon. Fuller details are on the way, including an interview with one of the team members from UAB behind this work and a publication based on the deliverable, which will be made available in the Outputs section of this site once published.